Ask Science
Why do apples turn brown? Could Jurassic Park really happen? Ask Science will help you make sense of the science in your everyday life.
Listen Now
More From Ask Science
Some of my best episodes, in my humble opinion, are inspired by the questions that my daughter asks me. To make it through another heatwave here in so...
Flossing every day, running more, eating more vegetables—whatever our goals, around four in ten of us will make resolutions to improve ourselves i...
Is astrology real? Reading horoscopes is a popular diversion, but is there any science to suggest it means anything? Inspiration finds you if you’re...
As of December 4, 2019, 565 people from 41 countries have gone into space. That’s it. 565 out of more than 7 billion of us currently on this plane...
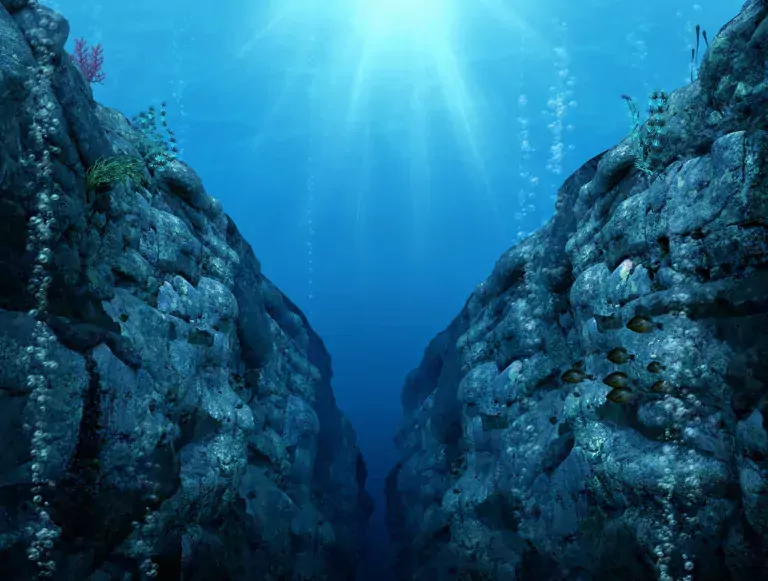
Somewhere between Hawaii and the Philippines near the small island of Guam, far below the surface of the water, sits the Mariana Trench, the deepest s...
Mariah Carey, Ella Fitzgerald, Bing Crosby, Mozart, Beethoven, Jimi Hendrix, and Yanni. What do these musicians have in common? They’re all said...
Eerie images from the site of the Chernobyl nuclear disaster still haunt us 30 years later. What is Chernobyl like today? On April 26, 1986, a safety ...
A stronger immune system, more energy, improved endurance, and better stamina … one ingredient promises all of that. Whether it’s as an extract, a...
As a parent, I often find myself asking questions I never thought I would ask: How long can someone survive on raisins alone? How did that yogurt get ...
Meteorologists talk not only about what the temperature will be outside, but also what that temperature “feels like.” Why are these numbers someti...
About
Why do apples turn brown? Could Jurassic Park really happen? Ask Science will help you make sense of the science in your everyday life.

.png)